Transvaginal Mesh Lawyer
Learn how Transvaginal Mesh works
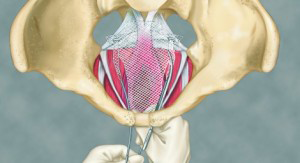 Transvaginal mesh is a net-like implant usually made of plastic called polypropylene. The term “transvaginal” refers to the type of surgical technique used to implant the mesh – through the vagina.
Transvaginal mesh is a net-like implant usually made of plastic called polypropylene. The term “transvaginal” refers to the type of surgical technique used to implant the mesh – through the vagina.
This type of surgical mesh was created to permanently fix pelvic organ prolapse (POP) and stress urinary incontinence (SUI) – conditions that typically plague women after a hysterectomy, menopause or childbirth. Pelvic organ prolapse occurs when a woman’s pelvic muscles weaken and the pelvic organs — including the bladder, rectum and uterus — drop into the vagina.
Doctors can surgically place the mesh transvaginally or abdominally, but inserting the mesh through the vagina is quicker, easier and less invasive.
While these products were created to help women suffering from SUI and POP, not all of them are safe and reliable. The design and manufacturer-recommended implantation technique of some of these transvaginal mesh products contributed to serious complications such as infection, erosion of the vaginal tissues and organ perforation.
Reports of complications came too late for hundreds of thousands of women who already had mesh implanted. Nearly 23,000 lawsuits filed by women injured by mesh are pending in the U.S. District Court. Additional lawsuits are pending in state courts.
Transvaginal Mesh
History of Transvaginal Mesh
Transvaginal mesh products evolved from surgical mesh originally used for hernia repair in the 1950s. Surgeons began using the mesh abdominally to repair POP and SUI in the 1970s before products were developed specifically to treat these conditions. The doctors took a piece of surgical mesh and cut the desired shape and size for use in each patient, then surgically implanted it.
Medical device manufacturers took notice of this clinical practice and responded by creating mesh products specifically designed to treat POP and SUI.
Boston Scientific manufactured the first transvaginal mesh device, called the ProtoGen Sling. In 1996, the U.S. Food and Drug Administration (FDA) approved the device for the surgical treatment of SUI through the 510(k) premarket program. However, just three years after the product was released, it was recalled over safety concerns.
In 1998, the use of mesh for SUI repair – also referred to as slings or tape – became more common with the release of Ethicon’s Tension-Free Vaginal Tape (TVT). The first surgical mesh product specifically designed for POP repair – Gynemesh PS – was released in 2002 by Johnson & Johnson’s Ethicon unit.
As the usage of these products became more common, manufacturers began selling their mesh products in “kits.” These kits are prepackaged with mesh, special tools and instructions to help doctors implant it.
The first kits for SUI were cleared for sale in 1997, followed by the first POP kits – the AMS Apogee and AMS Perigee, both manufactured by American Medical Systems.
Some doctors, like urogynecologist Dr. Christopher Walker, believe that the introduction of these kits contributed to some of the problems with transvaginal mesh.
Transvaginal Mesh Conditions
Learn about problems with Transvaginal Mesh
Transvaginal mesh is used to treat various health conditions cause by weakened pelvic muscles. The FDA categorizes mesh products into four categories.
› Non-absorbable synthetic. This type of mesh is considered a permanent implant because it will remain in the body indefinitely. More than half of all mesh products approved by the FDA fall into this category. These products are made from synthetic materials like plastic or polyester. Polypropylene is the most popular material for manufacturers, and 91 percent of non-absorbable synthetic mesh is made of this plastic.
› Absorbable synthetic. Absorbable mesh loses strength and degrades over time and is not intended as a long-term treatment. Ideally, the patient’s new tissue growth at the implant site helps to keep the repair strong.
› Biologic. These mesh products are natural and derived from animal tissue that has been specially disinfected for implanting in the human body. These products degrade over time and are usually made from cow (bovine) or pig (porcine) tissue.
› Composite. This mesh is made from a combination of any of the above three categories.
Manufacturers weave synthetic mesh materials together in several different shapes, depending on their intended use. When the fibers are woven together, pores are created on the surface of the material. According to the FDA, lightweight, large-pore mesh reduces the body’s inflammatory response.
Transvaginal Mesh Attorney
Speak with a Transvaginal Mesh Attorney Today
For a free case evaluation, contact our experienced Houston Transvaginal Mesh Attorney at Gary S. Tucker and Associates by calling 1-800-275-5007. We will answer any questions you may have regarding an Transvaginal Mesh lawsuits.
Areas for Houston Transvaginal Mesh Lawsuits:
77002, 77003, 77004, 77005, 77006, 77007, 77008, 77009, 77010, 77011, 77012, 77013, 77014, 77015, 77016, 77017, 77018, 77019, 77020, 77021, 77022, 77023, 77024, 77025, 77026, 77027, 77028, 77029, 77030, 77031, 77032, 77033, 77034, 77035, 77036, 77037, 77038, 77039, 77040, 77041, 77042, 77043, 77044, 77045, 77046, 77047, 77048, 77049, 77050, 77051, 77053, 77054, 77055, 77056, 77057, 77058, 77059, 77060, 77061, 77062, 77063, 77064, 77065, 77066, 77067, 77068, 77069, 77070, 77071, 77072, 77073, 77074, 77075, 77076, 77077, 77078, 77079, 77080, 77081, 77082, 77083, 77084, 77085, 77086, 77087, 77088, 77089, 77090, 77091, 77092, 77093, 77094, 77095, 77096, 77098, 77099, 77201, 77336, 77338, 77339, 77345, 77346, 77357, 77365, 77373, 77375, 77377, 77379, 77386, 77388, 77396, 77401, 77406, 77407, 77429, 77433, 77447, 77449, 77450, 77477, 77478, 77484, 77489, 77493, 77494, 77498, 77503, 77504, 77506, 77520, 77530, 77532, 77536, 77546, 77547, 77571, 77587, 77598.
